First HPV Self-Tests Now Available in US as Pap Smear Alternatives
An alternative to conventional cervical screening—otherwise known as smear tests or Pap smears—is now available and may help more women detect the human papillomavirus (HPV) before it becomes cervical cancer.
On Monday, the first self-collection tests approved by the Food and Drug Administration (FDA) were shipped to laboratories in the U.S., with more to come for health care facilities.
Cervical cancer is the fourth most common cancer in women globally. The World Health Organization (WHO) estimates there were 660,000 cases and 350,000 deaths associated with the disease in 2022.
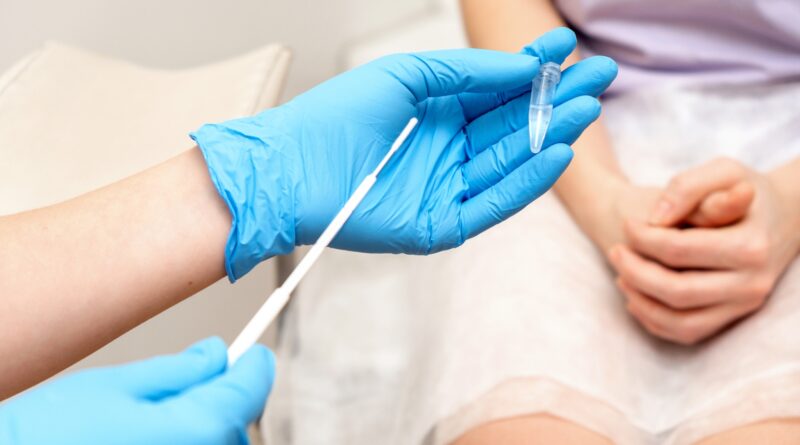
“Roche’s HPV self-collect solution allows women or people with a cervix to privately collect a sample with a swab or a brush in a health care setting,” Vanessa Bennett, senior international business leader at Roche, told Newsweek. Roche is a biotechnology company that produces the self-tests, along with medical technology company BD.
“Self-collection is when the sample is taken by the person who is being tested in a health care setting,” Bennett said. The patient would receive instructions on carrying out the test privately, and a health care worker could be nearby to help if needed.
“The test involves inserting a swab about 3 inches into the vagina and collecting a sample of cells to be tested for HPV,” she said.
Approximately 95 percent of cervical cancer cases are caused by persistent infections with HPV, according to the WHO. Cervical screening can help doctors identify women who are at risk of cervical cancer so they can detect HPV and treat the disease early.

Tatiana Buzmakova/Getty Images
The National Cancer Institute estimates that more than half of cervical cancer cases in the U.S. are among women who have never been screened or have been infrequently screened for HPV.
“Many factors can contribute to individuals not participating in cervical cancer screening programs, such as access to health care, history of traumatic experience, cultural concerns and embarrassment,” Bennett said. “HPV self-collection can broaden access to care when a cervical specimen may not be available.”
The American Cancer Society recommends HPV testing every five years for people with cervices and aged 25 to 65.
“Many patients are uncomfortable with the intimate nature of a pelvic exam,” said BD gynecologist Jeff Andrews in a statement. “Also, many people live in areas without a local doctor or clinician trained to obtain a sample with a speculum. The option to self-collect in a clinical setting can help women overcome some of these barriers.”
The FDA approved the HPV self-collection tests from Roche and BD in May, but they have only just become available for patients.
“People should ask their clinician about HPV self-collection and whether it’s right for them,” Bennett said. “Some clinicians may choose to have self-collection on hand for patients who are appropriate for it.
“Also, public health clinics may be interested in offering HPV self-collection as another screening option to reach under-screened and never screened patients. Ultimately, access will depend on clinicians offering the option and labs having the capacity,” she said.
Do you have a tip on a food story that Newsweek should be covering? Is there a nutrition concern that’s worrying you? Let us know via science@newsweek.com. We can ask experts for advice, and your story could be featured in Newsweek.